SLIDE CULTURE TECHNIQUE
AIM
To identify the Fungi morphology
without any disturbance by Slide culture technique.
PRINCIPLE
Fungi are the group of
Eukaryotic microorganisms and they are identified mostly by close examination
of its morphology and the characteristics it possess. Identification of Fungi
is often difficult by tease mount method because of the dislodgement of conidia
and spores from the Conidiogenous cell. To overcome this, Slide cultures
technique is considered best for preserving and observing the actual structure
of a fungus without any disturbances. The method was first developed by Riddel
in 1950 and currently, several modifications are in use.
In Slide cultures technique, fungi
are inoculated in small blocks of Nutrient deficient agar medium (Cornmeal agar
or Potato dextrose agar) (Fungi when grown in nutrition deficient medium
develop spores rapidly and adhere to the surface of the coverslip), covered
with a coverslip and incubated. After incubation, the coverslip is removed from
the agar block and placed on another slide to which a dye, such as Lactophenol
cotton blue (LPCB), may be added and observed for microscopic structures. By
doing this, there is no need to remove a portion of the fungus from a culture
plate and transfer it to the slide. So, there is less chance for the features
that are key to identification, notably the spore-bearing structures, to be
damaged.
MATERIALS REQUIRED
- Fungal culture
- Cornmeal agar or Potato dextrose agar
- Petridish
- Scalpel or Spatula
- Glass slide
- Cover slip
- Lactophenol cottonblue (LPCB) stain
PROCEDURE
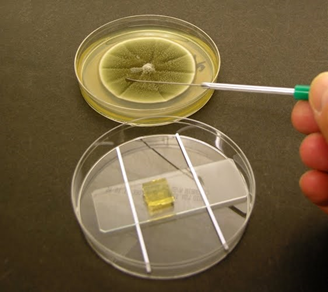
a) Prepare the Cornmeal
agar or Potato dextrose agar medium and cut the block of agar (7 × 7 mm)
by inserting the scalpel and carefully transfer this block aseptically to the
centre of the Glass slide.
b) Inoculate four sides of the agar square with spores or
mycelial fragments of the fungus to be examined. Be sure to flame and cool the
loop prior to picking up spores.
c) Aseptically, place a sterile Cover slip on the upper
surface of the agar cube.
d) Place the Glass slide with Fungi inoculated agar block
on the Petridish with wet cotton on both sides and incubate at room temperature
for 48 hours.
e) After 48 hours, gently remove the Cover slip from the
fungi inoculated Agar block and place it on a microscope slide containing a
drop of Lactophenol cotton blue.
f) Observe microscopically for the characteristic shape
and arrangement of spores.
Figure – 1: Slide culture technique
OBSERVATION AND RESULTS
The morphology of the fungi was observed clearly
without any disturbance under microscope in 45 X objective.




Comments
Post a Comment